-
2020

Participating in drawing up CP2025 monographs
◎Participating in drawing up of Povidone K25, K90 in CP2025 monographs
-
2019
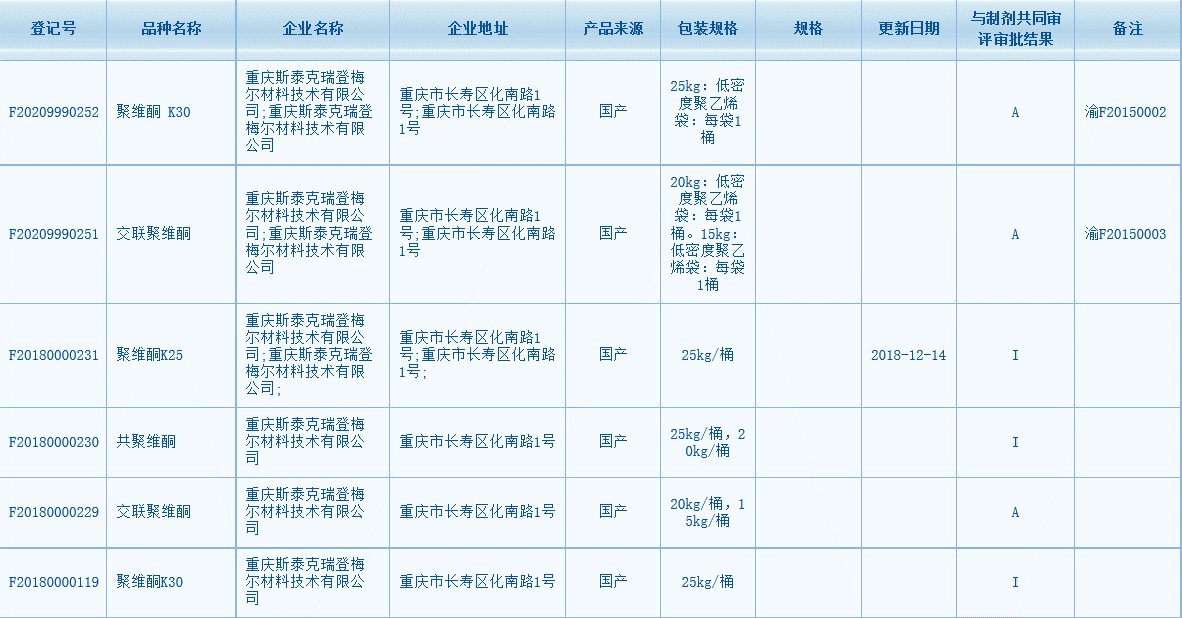
Participating in revision work in CP2020
◎Participating in revision of Povidone and Crospovidone, and drawing up of Copovidone in CP2020 monographs
◎Crospovidone to be "Active" in CDE registration
-
2018

A joint R&D Center of Innovative Technology For PVPs
◎A joint R&D Center of Innovative Technology For PVPs with Chongqing University, one of the best universities in China
◎Chongqing Municipal Enterprise Technology Center -
2017

EXCiPACT GMP Certificate
◎EXCiPACT GMP Certificate◎Chongqing Municipal High-Tech Enterprise -
2016

A joint-venture agreement with JRS Germany
◎A joint-venture agreement with JRS Germany, a leading pharm excipient producer in the world◎“Food Production License” from CFDA -
2015

CEP from EDQM
◎CEP for Povidones, Copovidone and Crospovidone from EDQM◎Registration Certificates for Povidone K30 and Crospovidone from CFDA -
2014

Pharmaceutical Production License
◎“Pharmaceutical Production License" From CFDA◎GMP Audit Certificate issued by an independent European consultant◎ISO9001, ISO14001, OHSAS18001, HACCP certificates◎US-DMF for Povidone, Copovidnoe and Crospovidone -
2013

Construction phase
◎"Quality by Design"
◎Building up as per cGMP guidelines
-
2012

PVP project approval
◎PVP project approval◎Innovative technologies
◎QMS and EHS systems


