-
10
2020/04
Participating in drawing up CP2025 monographs
-
10
2020/04
Participating in revision work in CP2020
-
02
2019/04
A joint R&D Center of Innovative Technology For PVPs
-
02
2019/04
EXCiPACT GMP Certificate
-
28
2018/03
A joint-venture agreement with JRS Germany
-
09
2020/04
EXCiPACT GMP Certificate
-
28
2018/03
CEP from EDQM
-
10
2023/05
EDQM CEP - POVIDONE K25, K30
-
06
2021/05
EDQM CEP - POVIDONE K90
-
09
2020/04
EDQM CEP - COPOVIDONE
-
09
2020/04
EDQM CEP - CROSPOVIDONE Type A & Type B
-
28
2018/03
Pharmaceutical Production License
-
02
2024/08
ISO 22000:2018
-
02
2024/08
ISO 45001:2018
-
02
2024/08
ISO 14001:2015
-
09
2020/04
ISO 9001:2015
-
28
2018/03
Construction phase
-
13
2021/08
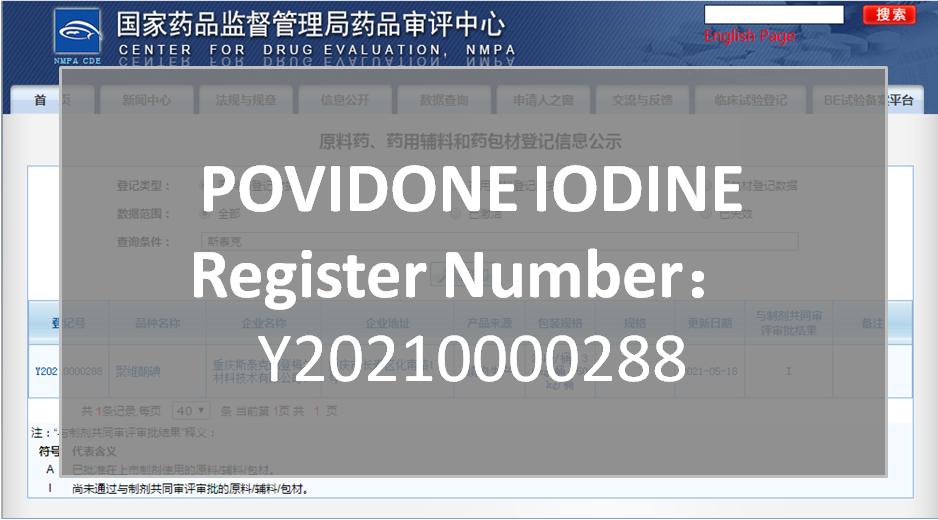
NMPA CDE registration - POVIDONE IODINE
-
18
2021/01
NMPA CDE registration - POVIDONE K90
-
26
2020/04
CPEC certificate
/index.php?c=article&a=type&tid=8